Hear from a pediatric endocrinologist
Amrit Bhangoo, MD, explains why he prescribes VOXZOGO as early as birth.1
Over 1 year in the phase 3 pivotal trial (Study 301), adverse reactions that occurred in ≥5% of patients aged 5 to 15 years treated with VOXZOGO and at a percentage greater than placebo are provided in the table.1
In the long-term open-label extension study (Study 302), adverse reactions reported for up to 6 years of treatment are consistent with those seen in the 1-year pivotal trial.2
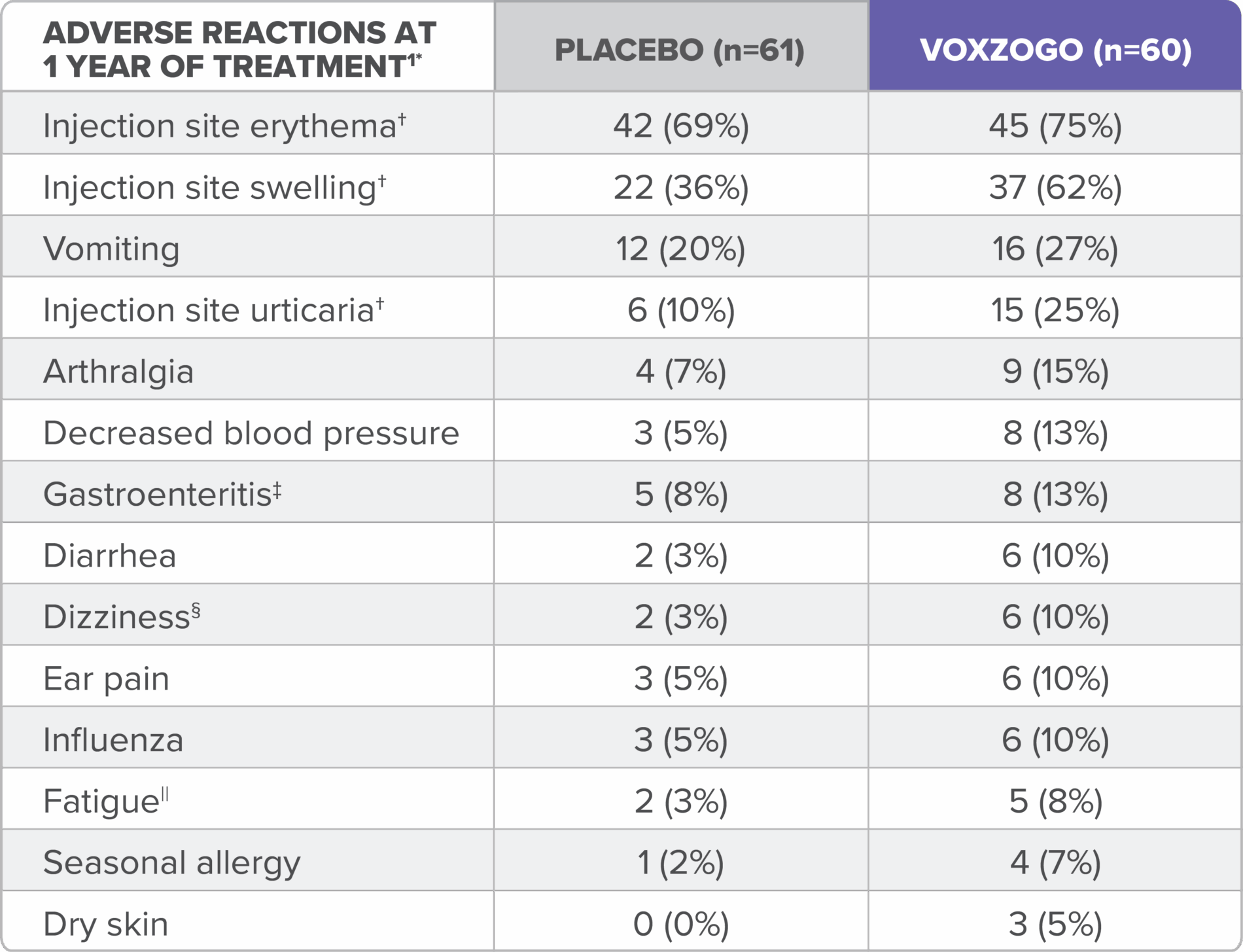
*Includes adverse reactions occurring more frequently in the VOXZOGO arm and with a risk difference of ≥5% (ie, difference of >2 subjects) between treatment arms.1
†Injection site reactions occurring more frequently in VOXZOGO-treated patients than placebo.1
‡Includes the preferred terms: gastroenteritis and gastroenteritis, viral.1
§Includes the preferred terms: dizziness, presyncope, procedural dizziness, and vertigo.1
||Includes the preferred terms: fatigue, lethargy, and malaise.1
Over 1 year in the phase 2 trial (Study 206), the most common adverse reactions (>10%) reported in patients <5 years were injection site reactions (86%) and rash (28%).1
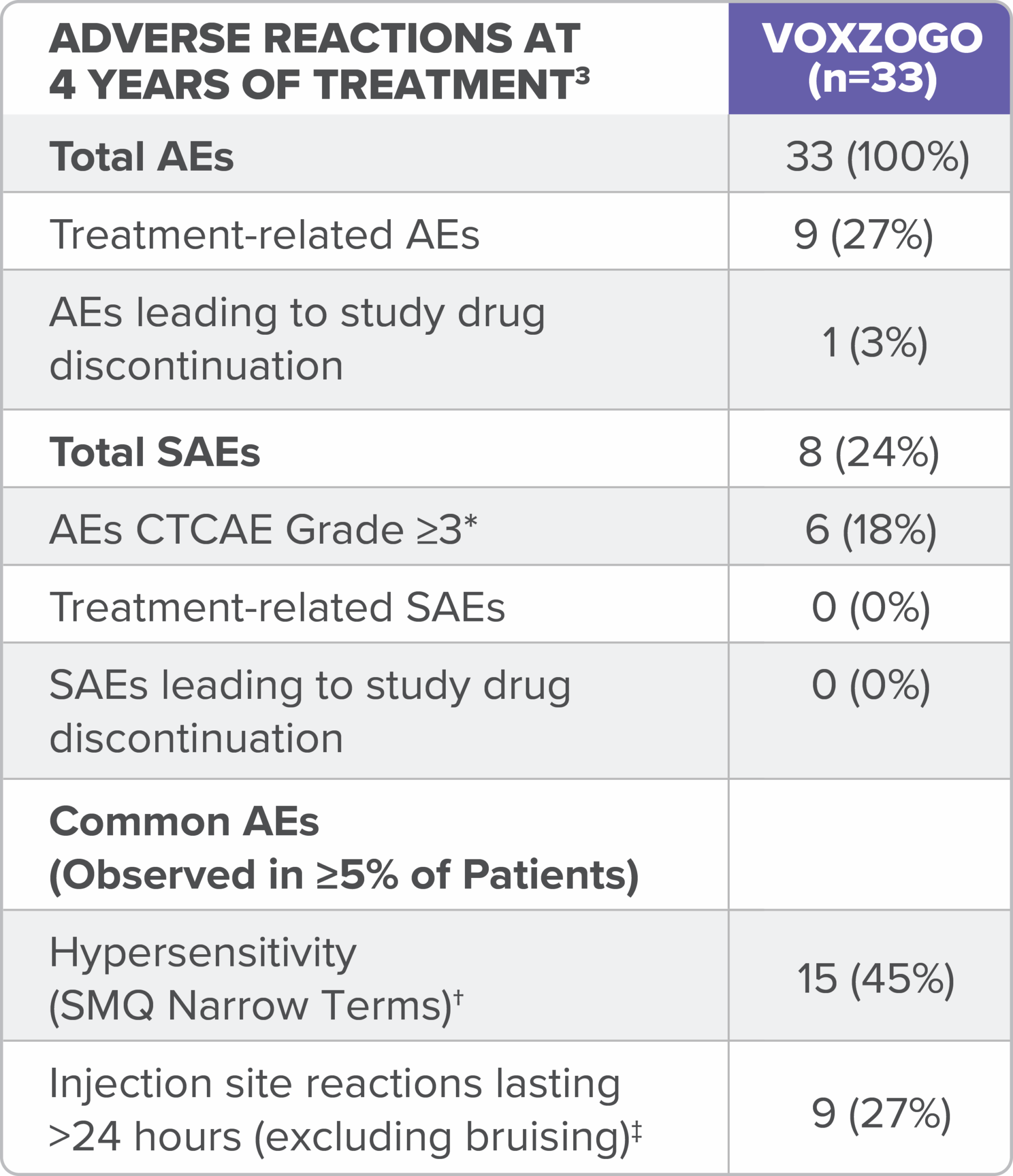
In the long-term open-label extension study (Study 208), adverse reactions reported for up to 4 years of treatment are consistent with those seen in the 1-year trial (see table).3
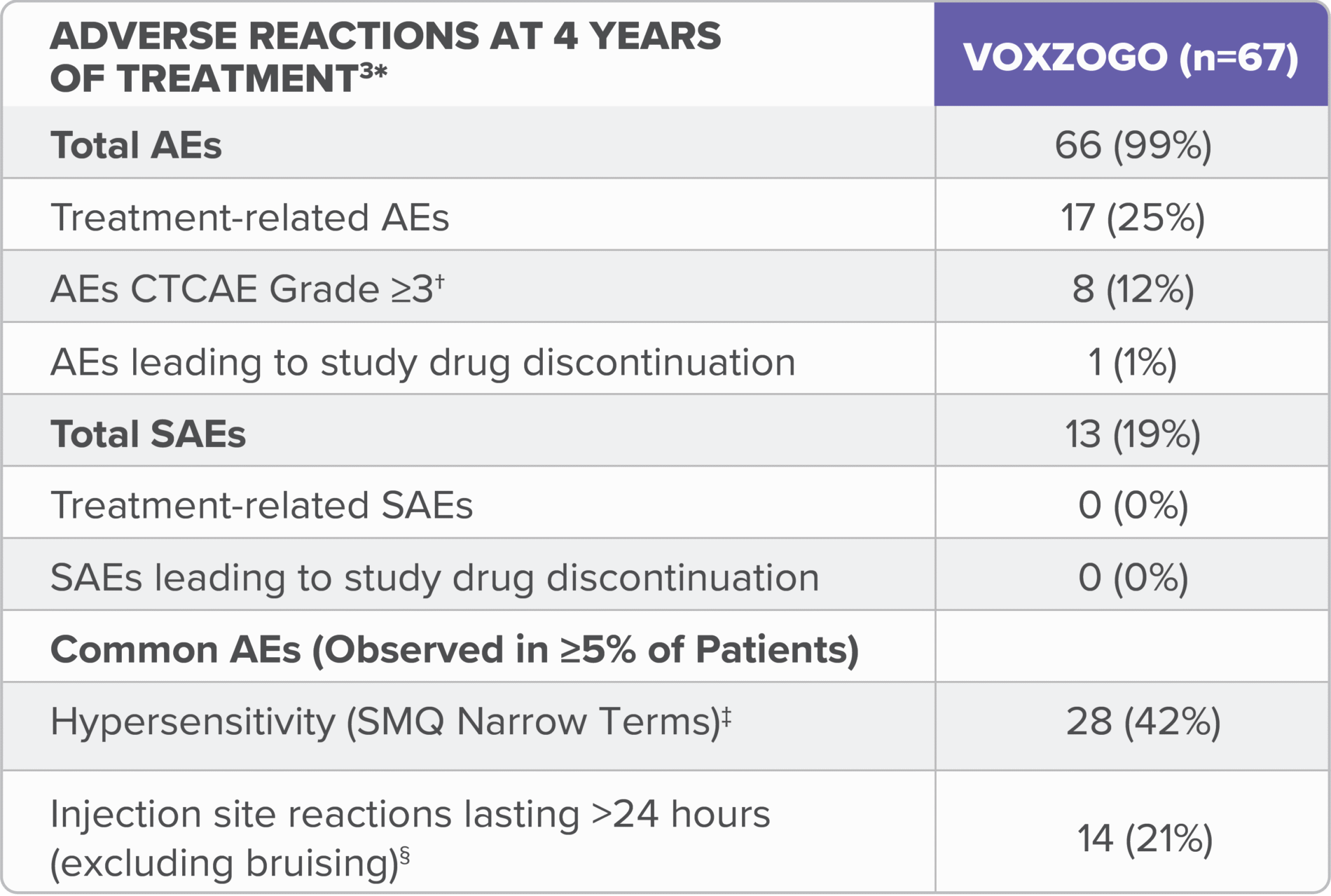
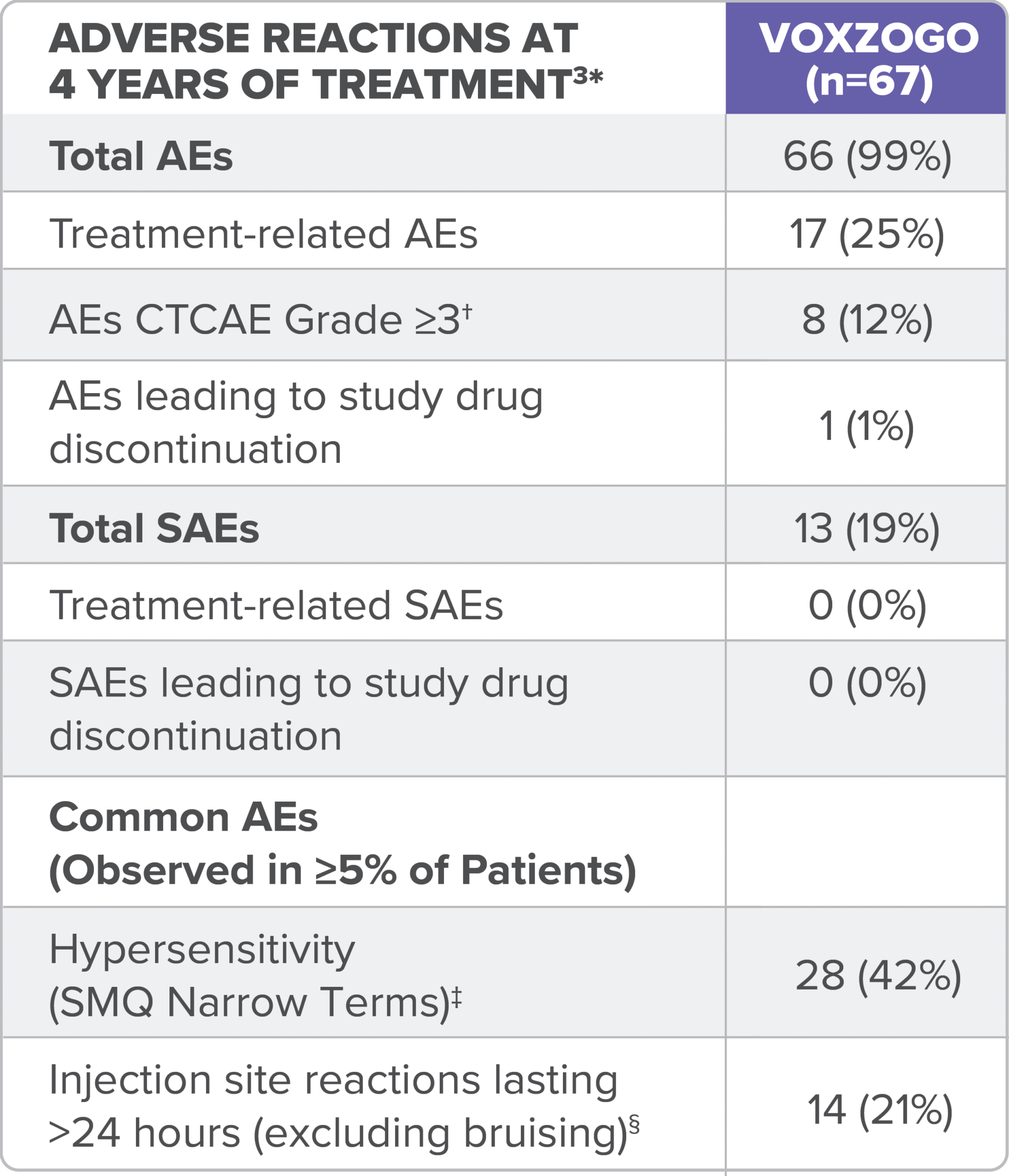
ADL, activities of daily living; AE, adverse event, CTCAE, Common Terminology Criteria for Adverse Events; SAE, serious adverse event.
*Data combined from children aged <2 years and 2 to <5 years at the start of treatment in clinical trials.3
†CTCAE (v5.0) grades refers to the severity of the AE: Grade 1 = mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated; Grade 2 = moderate; minimal, local, or non-invasive intervention indicated; limiting age-appropriate instrumental ADL; Grade 3 = severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care ADL; Grade 4 = life-threatening consequences; urgent intervention indicated; Grade 5 = death related to AE.4
‡No CTCAE Grade ≥3 hypersensitivity or anaphylaxis reported.3
§No CTCAE Grade ≥2 injection site reactions were reported.3
Over 4 years in the in the long-term open-label extension study (Study 208), adverse reactions reported in children <2 years old at treatment initiation (see table) were consistent with those seen in children aged 2 to <5 years old.3
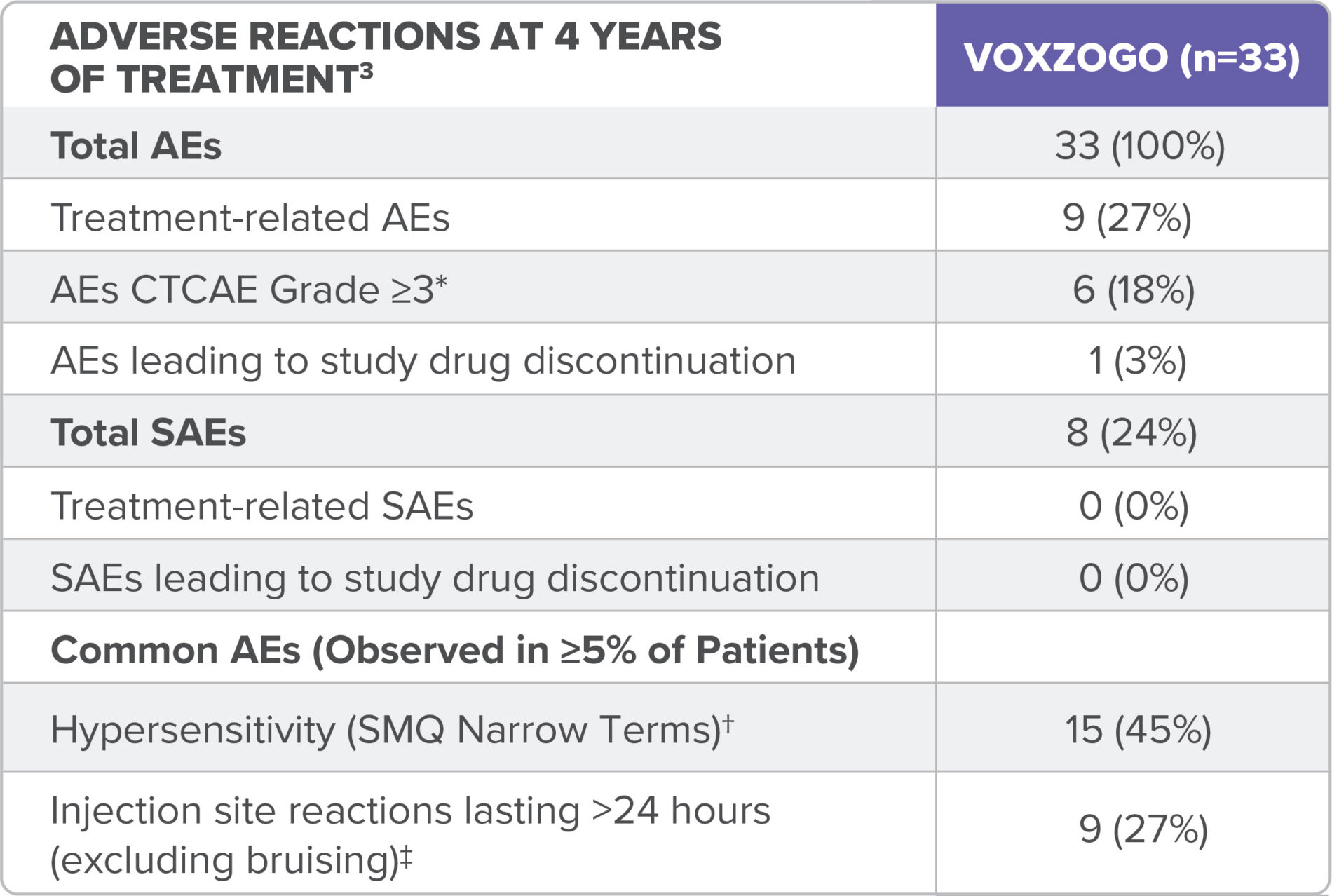
ADL, activities of daily living; AE, adverse event, CTCAE, Common Terminology Criteria for Adverse Events; SAE, serious adverse event.
*CTCAE (v5.0) grades refers to the severity of the AE: Grade 1 = mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated; Grade 2 = moderate; minimal, local, or non-invasive intervention indicated; limiting age-appropriate instrumental ADL; Grade 3 = severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care ADL; Grade 4 = life-threatening consequences; urgent intervention indicated; Grade 5 = death related to AE.4
†No CTCAE Grade ≥3 hypersensitivity or anaphylaxis reported.3
‡No CTCAE Grade ≥2 injection site reactions were reported.3
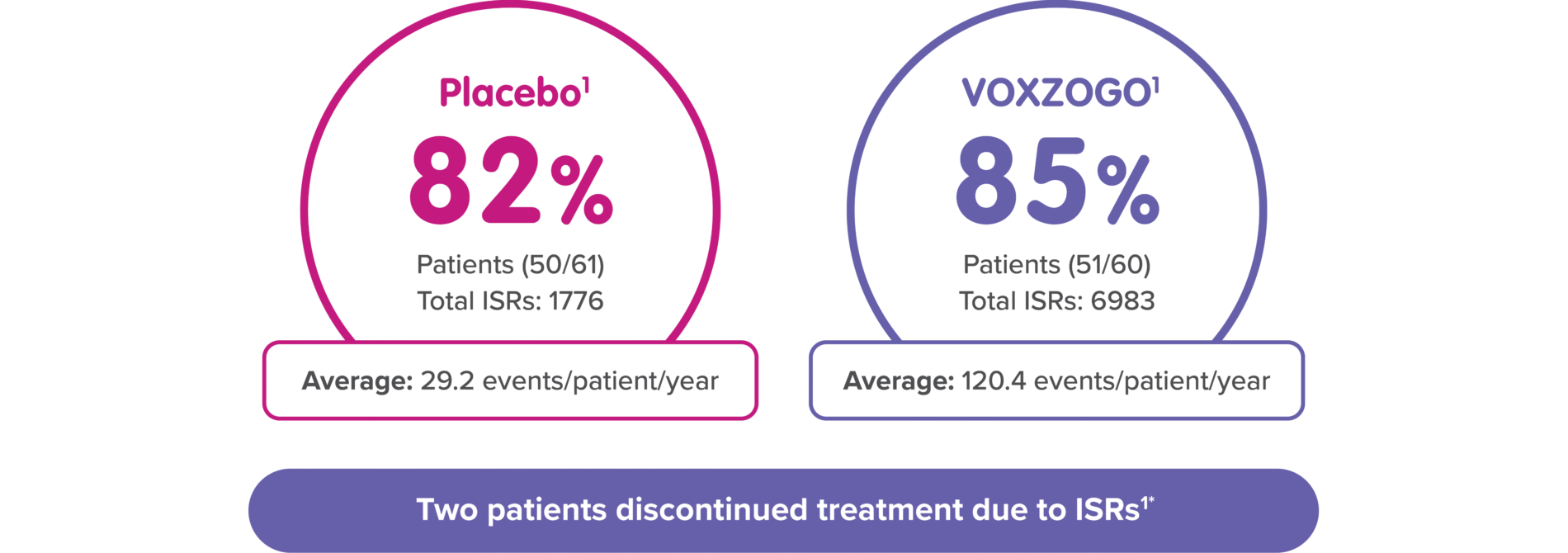
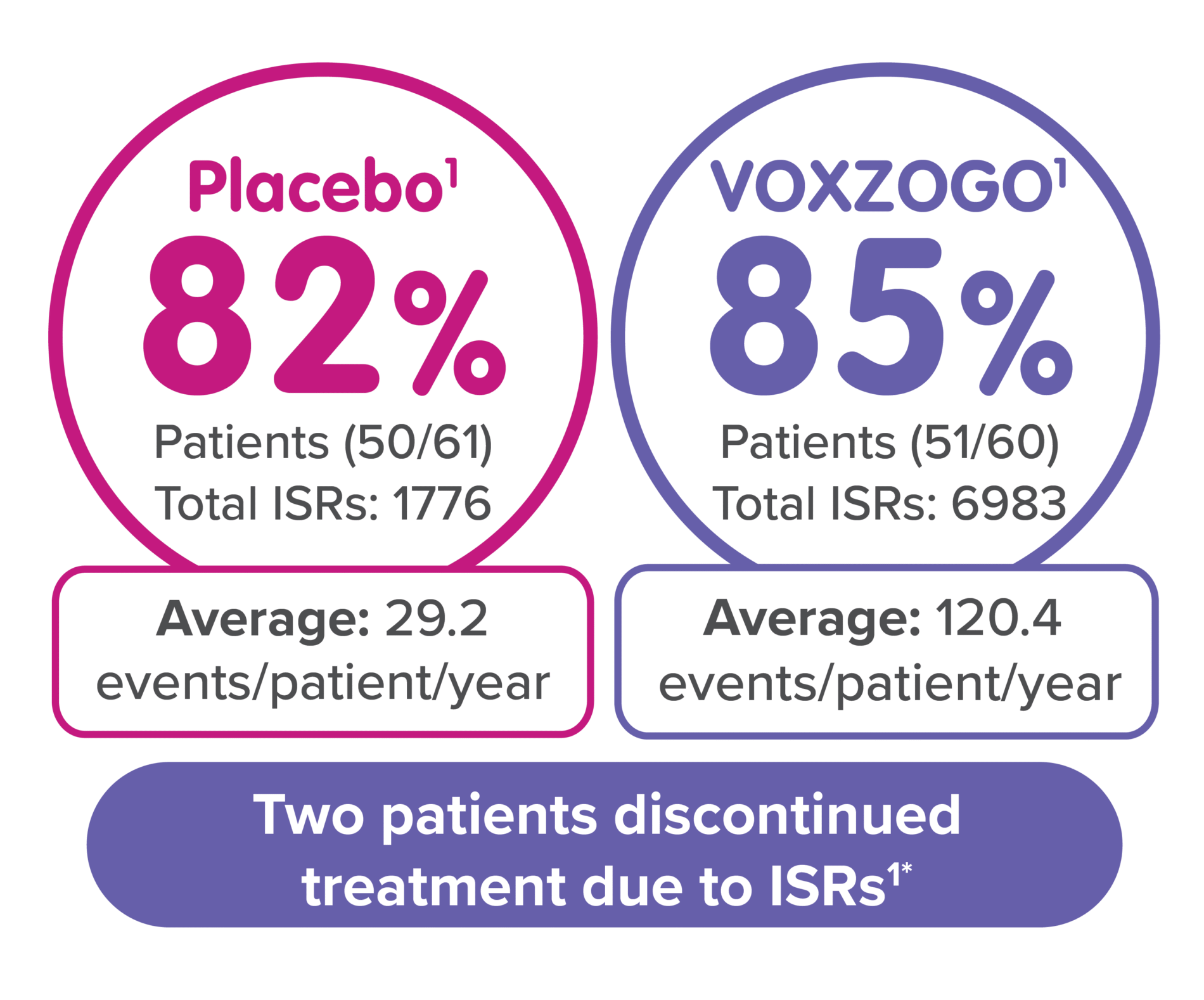
*One patient discontinued treatment due to pain, and one discontinued treatment due to anxiety with injections.1
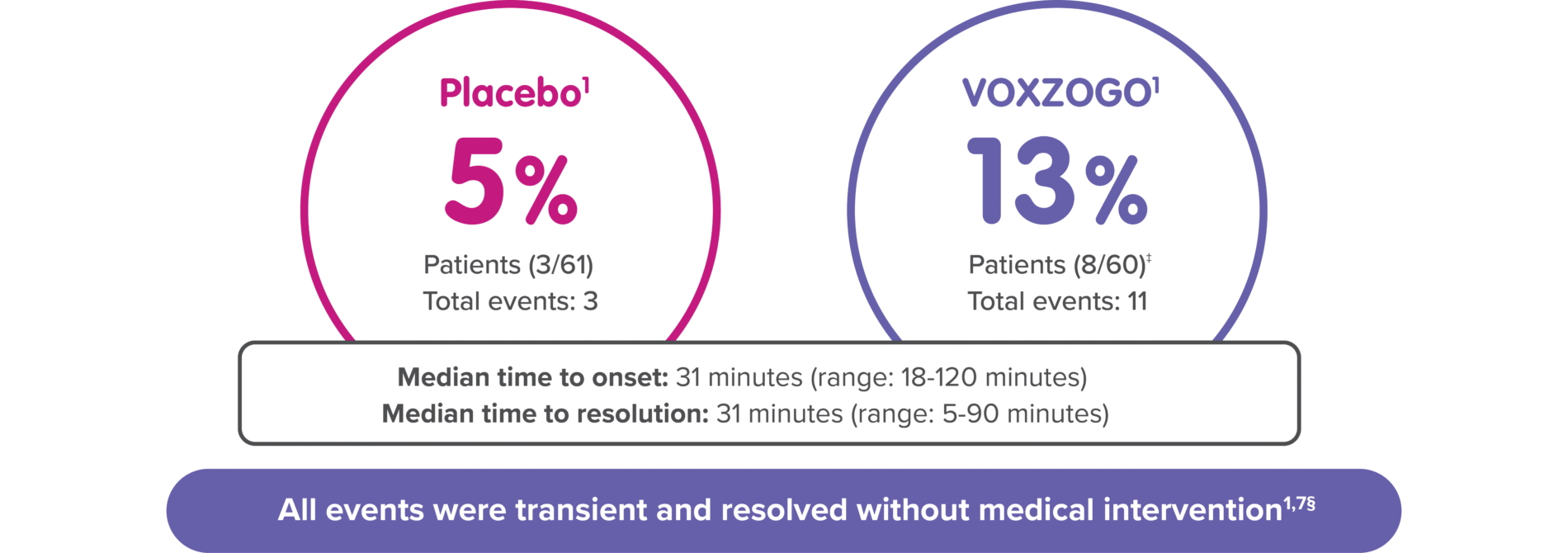
VOXZOGO may cause serious side effects including a temporary decrease in blood pressure in some patients; to reduce the risk of a decrease in blood pressure and associated symptoms (dizziness, feeling tired, or nausea), patients should be well fed and hydrated in the hour before receiving VOXZOGO.1
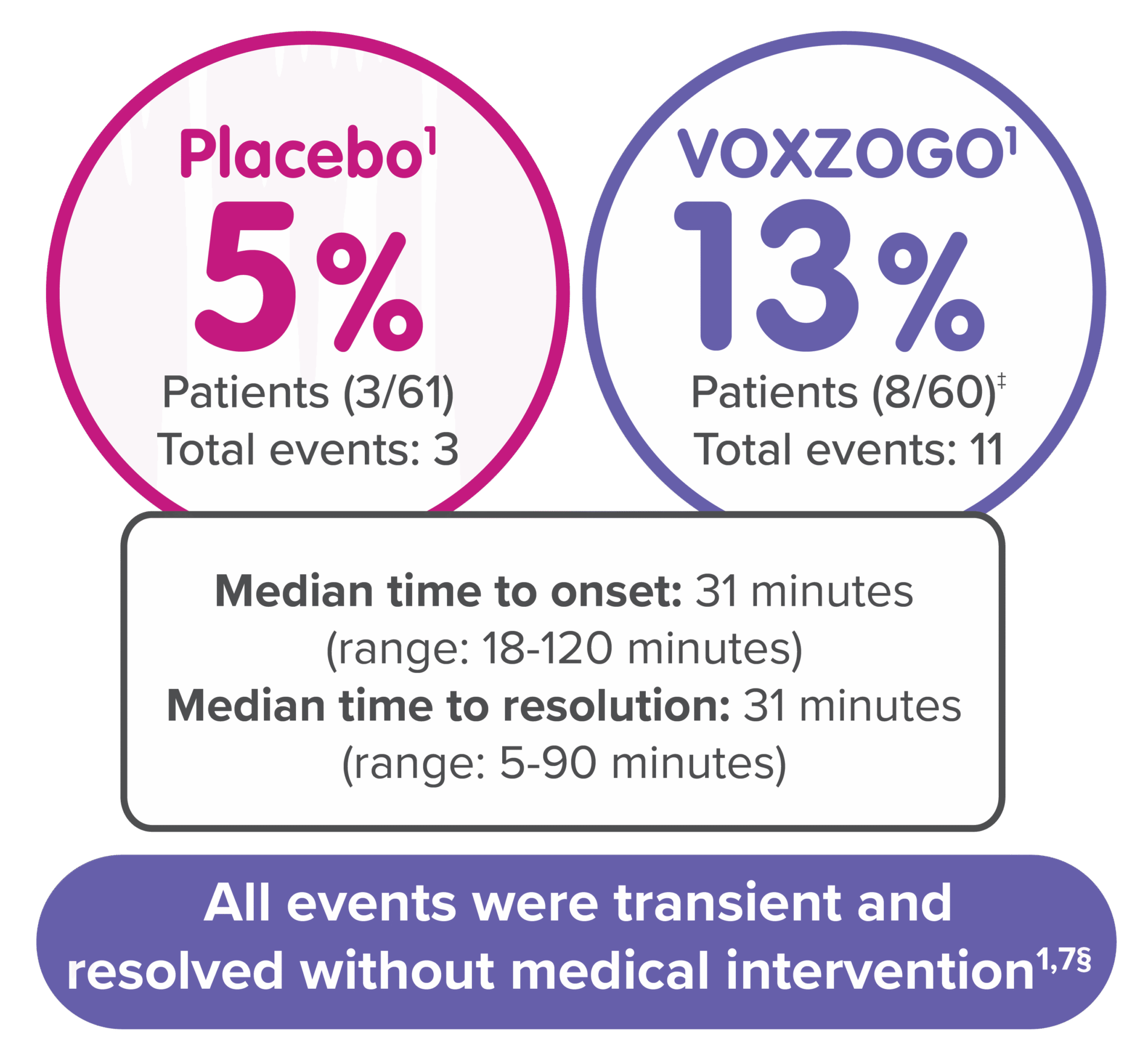
†Decreased blood pressure includes blood pressure decrease and hypotension; changes were identified mainly during periods of frequent vital sign monitoring at clinical visits after dosing over 1 year of treatment.1
‡3% (2/60) of patients on VOXZOGO each had 1 symptomatic decreased blood pressure event with vomiting and/or dizziness vs 0% of patients on placebo.1
§Post-dose decreases in systolic blood pressure <70 mm Hg (+2x age): 23% (14/60) patients on VOXZOGO vs 25% (15/61) patients on placebo; post-dose decreases in diastolic blood pressure <40 mm Hg: 17% (10/60) patients on VOXZOGO vs 10% (6/61) patients on placebo.7
Amrit Bhangoo, MD, explains why he prescribes VOXZOGO as early as birth.1
Warnings and Precautions for Risk of Low Blood Pressure
Transient decreases in blood pressure were observed in clinical studies. Patients with significant cardiac or vascular disease and patients on anti-hypertensive medicinal products were excluded from participation in VOXZOGO clinical trials. To reduce the risk of a decrease in blood pressure and associated symptoms (dizziness, fatigue, and/or nausea), patients should be well hydrated, have adequate food intake, and drink approximately 8-10 ounces of fluid in the hour prior to VOXZOGO administration.
In a 52-week, randomized, double-blind, placebo-controlled trial in 121 subjects with achondroplasia, subjects aged from 5.1 to 14.9 years, (Study 1) eight (13%) of 60 patients treated with VOXZOGO had a total of 11 events of transient decrease in blood pressure, compared to 3 (5%) of 61 patients on placebo, over a 52-week treatment period. The median time to onset from injection was 31 (18 to 120) minutes, with resolution within 31 (5 to 90) minutes in VOXZOGO-treated subjects. Two out of 60 (3%) VOXZOGO-treated patients each had one symptomatic episode of decreased blood pressure with vomiting and/or dizziness compared to 0 of 61 (0%) patients on placebo.
Adverse Reactions:
Adverse reactions that occurred in ≥5% of patients treated with VOXZOGO and at a rate greater than that of placebo in the phase 3 study are injection site reactions (including erythema, swelling, urticaria, pain, bruising, pruritus, hemorrhage, discoloration, and induration), vomiting, arthralgia, decrease in blood pressure, gastroenteritis, diarrhea, dizziness, ear pain, influenza, fatigue, seasonal allergy, and dry skin. VOXZOGO-treated patients had an increase in alkaline phosphatase levels (17%), and was noted as a laboratory abnormality.
Injection site reactions: In Study 1, injection site reactions occurred in 51 (85%) subjects receiving VOXZOGO and 50 (82%) subjects receiving placebo over a 52-week period of treatment. Patients receiving VOXZOGO experienced a total of 6983 events of injection site reactions, while patients receiving placebo experienced a total of 1776 events of injection site reactions, over a 52-week period, representing 120.4 events per patient/year exposure and 29.2 events per patient/year exposure, respectively. Two patients in the VOXZOGO arm discontinued treatment due to adverse events of pain and anxiety with injections.
Pediatric Patients 0 to <5 Years:
The safety of VOXZOGO in pediatric patients 0 to <5 years with achondroplasia was evaluated in a 52-week randomized, double-blind, placebo-controlled study (Study 2). In this study, 64 patients from birth to <5 years of age were randomized to receive either a daily vosoritide dose with similar exposure to that characterized to be safe and effective in children with ACH aged ≥5 years old, or placebo. An additional 11 patients received open-label treatment as part of this study. The most common adverse reactions (>10%) reported in pediatric patients 0 to <5 years were injection site reactions (86%) and rash (28%). The overall safety profile of VOXZOGO in pediatric patients 0 to <5 years was similar to that seen in older pediatric patients.
Administration and Monitoring:
VOXZOGO is administered as a daily subcutaneous injection. Prior to use, instruct caregivers on proper preparation and administration of VOXZOGO, and ensure caregivers have demonstrated the ability to perform a subcutaneous injection.
Monitor and assess patient body weight, growth, and physical development regularly every 3-6 months. Adjust dosage according to the patient’s actual body weight. Permanently discontinue treatment with VOXZOGO upon confirmation of no further growth potential, indicated by closure of epiphyses.
Special Populations:
You may report side effects to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. You may also report side effects to BioMarin at 1-866-906-6100.
Please see additional safety information in the full Prescribing Information.
VOXZOGO® (vosoritide) is indicated to increase linear growth in pediatric patients with achondroplasia and open growth plates.