Most parents are of average stature, which means they may need your expertise to prepare them for the possible complications caused by impaired bone growth.4,17
Achondroplasia occurs in 1 out of 25,000 live births and affects around 250,000 people worldwide1,2 Achondroplasia accounts for nearly 90% of disproportionate short stature or dwarfism. Characterized by impaired endochondral bone growth, it is caused by a gain-of-function pathogenic variant in the fibroblast growth factor receptor 3 (FGFR3) gene and has distinct physical characteristics1,3-8
Physical characteristics, such as height, are indicators of bone growth throughout the body.
Endochondral ossification, the replacement of cartilage by bone, occurs throughout the body and is involved in the development of roughly 90% of all bones. This process begins in utero and continues into early adulthood.10-12
In endochondral ossification, the precursor to prospective bone is cartilage




The gain-of-function pathogenic variant causes FGFR3 to excessively generate signals to slow down bone growth, overwhelming the counteracting signaling from the NPRB/CNP pathway, and resulting in impaired bone growth.4,16


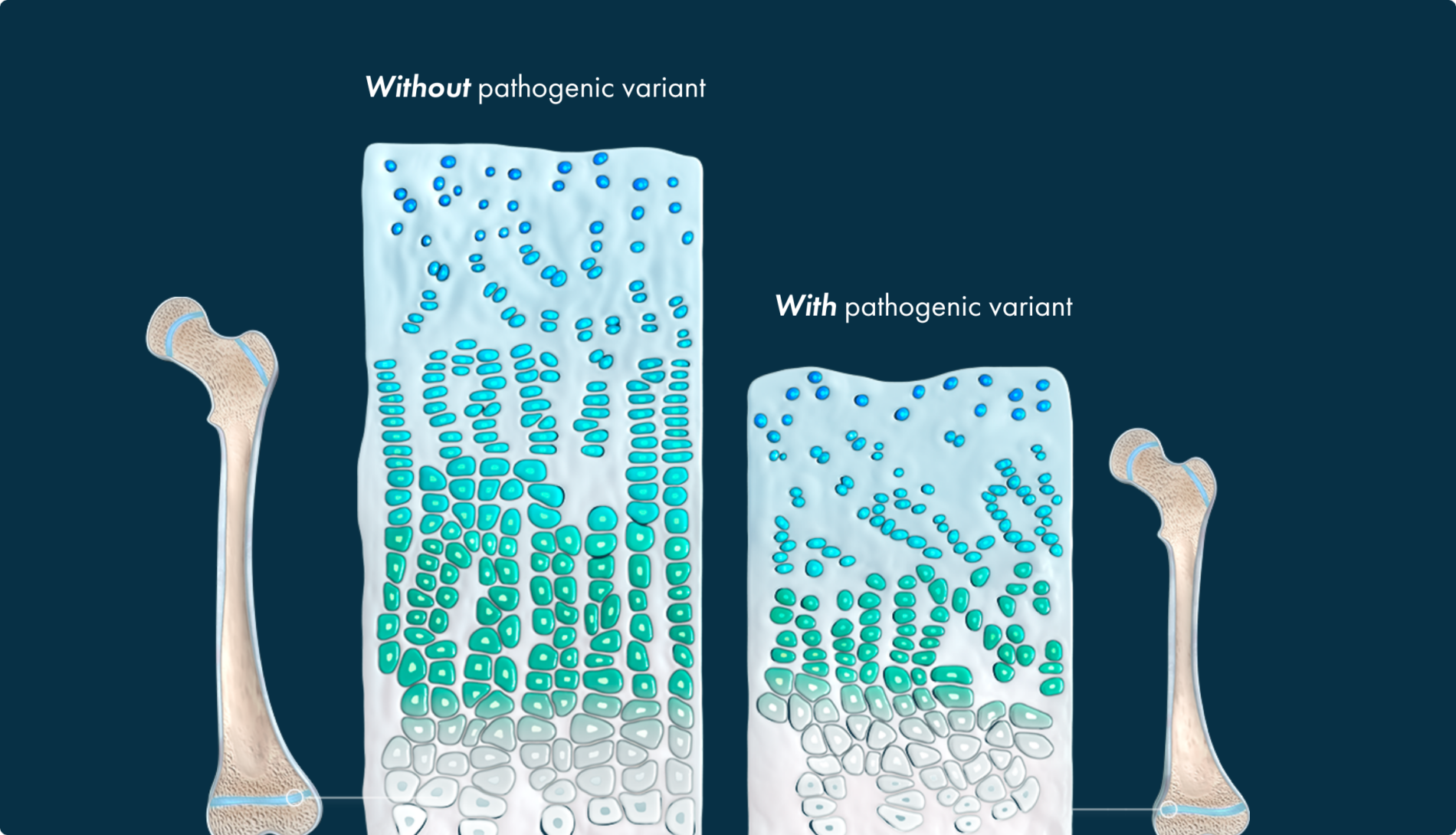
This leads to a multisystemic impact that parents may not be prepared for.
Most parents are of average stature, which means they may need your expertise to prepare them for the possible complications caused by impaired bone growth.4,17
Achondroplasia occurs in approximately _________ live births worldwide.

Common achondroplasia complications can be expected across an individual’s lifetime.

Everything you need to deepen your achondroplasia knowledge