Interested in learning more?
Connect with a BioMarin representative for a detailed presentation.




PALYNZIQ is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the PALYNZIQ REMS.
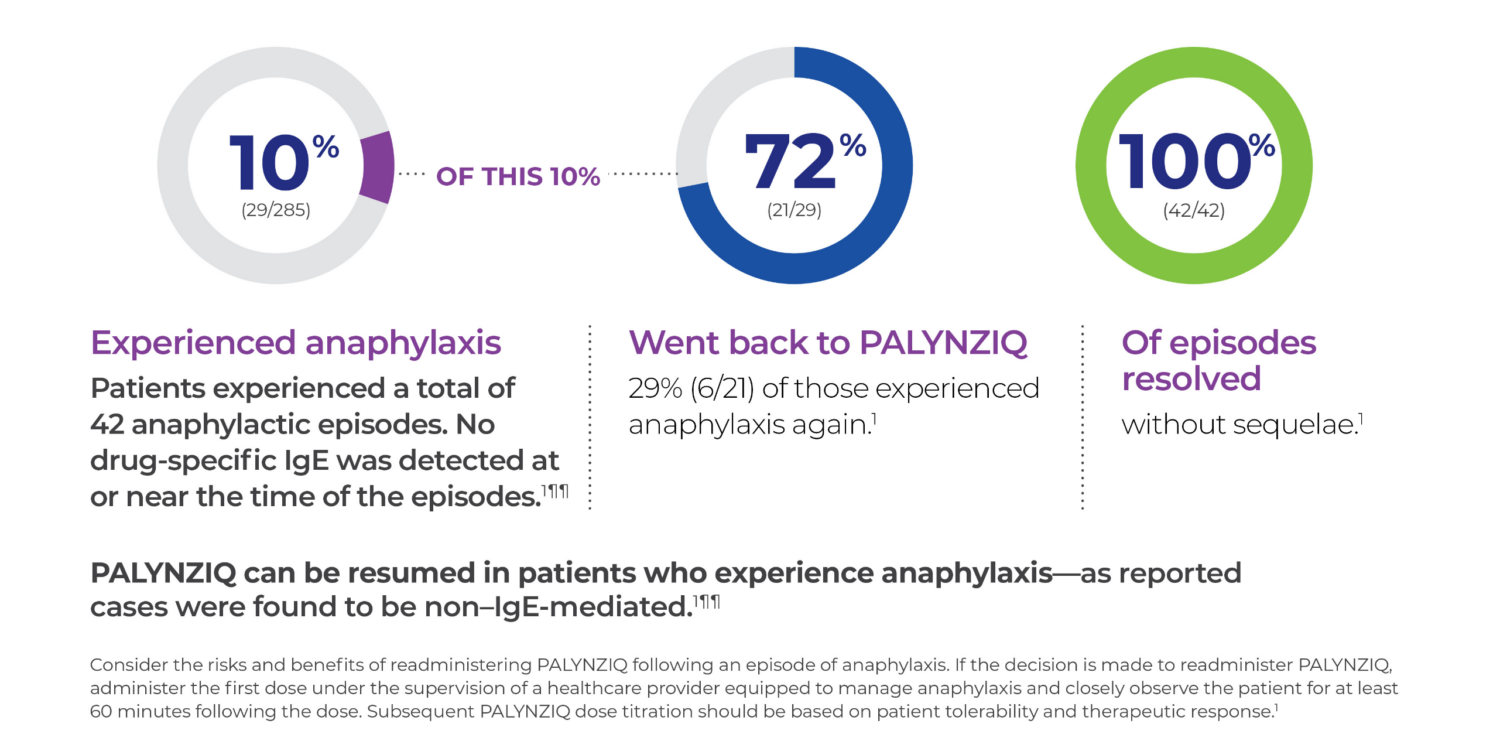
Hypersensitivity reactions other than anaphylaxis were reported in 204/285 (72%) patients. Management should be based on the severity of the reaction, recurrence, and clinical judgment, and may include dosage adjustment, temporary drug interruption, or treatment with antihistamines, antipyretics, and/or corticosteroids.1


The most common adverse reactions leading to discontinuation were hypersensitivity reactions (6%), including anaphylaxis (3%), angioedema (1%), arthralgia (4%), generalized skin reactions lasting at least 14 days (2%), and injection site reactions (1%).1



*Monitor patients’ dietary protein and phenylalanine intake throughout treatment with PALYNZIQ and counsel them on how to adjust their dietary intake, as needed, based on blood phenylalanine concentrations.
†Of the 261 patients who enrolled in Study 301, 54 (21%) patients discontinued treatment during Study 301, 4 patients completed Study 301 and did not continue to Study 165-302 (referred to as Study 302, NCT01889862], 152 patients continued to the eligibility period of Study 302, and 51 patients continued directly from Study 301 into the long-term treatment period of Study 302.1
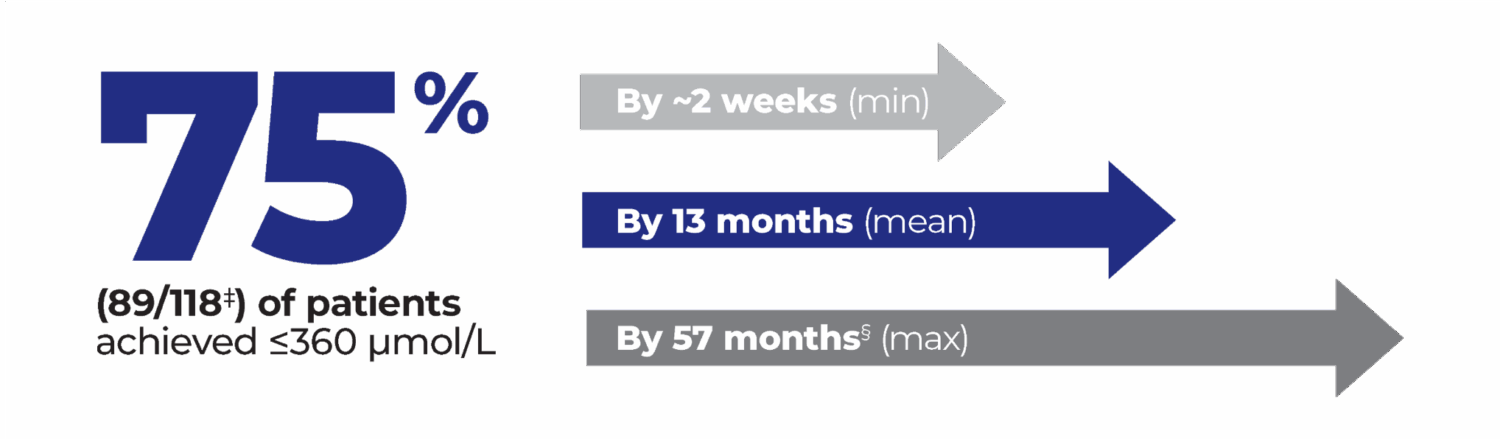
‡Of the 118 patients from Study 301 with a pre-treatment baseline blood phenylalanine concentration greater than 600 micromol/L who were randomized to and received at least one dose of 20 mg once daily, 21% (25/118) of patients discontinued the study prior to achieving ≤360 µmol/L. Of those, 10 patients discontinued due to adverse events. 3% (4/118) did not achieve ≤360 mmol/L before study completion.1,3
§The majority of those patients (86/89) achieved ≤360 µmol/L by 36 months.3
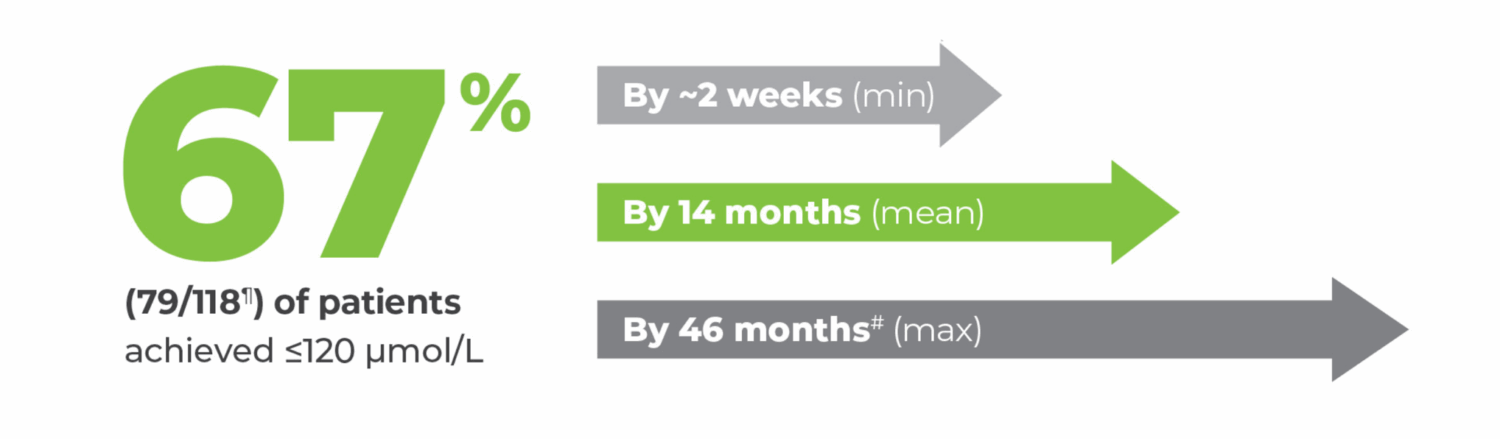
¶Of the 118 patients from Study 301 with a pre-treatment baseline blood phenylalanine concentration greater than 600 micromol/L who were randomized to and received at least one dose of 20 mg once daily, 24% (28/118) of patients discontinued the study prior to achieving ≤120 µmol/L. 11 patients did not achieve ≤120 µmol/L before study completion.1,3
#The 25th percentile of patients who achieved ≤120 µmol/L did so by 7 months; the 75th percentile, by 18 months.3
**Injection site reactions can include redness, itching, pain, bruising, rash, swelling, or tenderness.1
††Maintenance phase defined as when subjects reached stable dose for 8 weeks.1
‡‡Hypersensitivity, including anaphylaxis.1
§§Maintenance, all doses includes patients on placebo and PALYNZIQ <20 mg.1,5
¶¶27 of 29 patients who had anaphylaxis were tested for anti-pegvaliase-pqpz lgE antibodies, which recognize the PEGylated protein product. Of those 27 patients, 26 tested negative. The one patient who screened positive for anti-pegvaliase-pqpz lgE had insufficient sample to confirm lgE positivity. This patient tested negative for anti-pegvaliase-pqpz lgE at routine visits before and after the anaphylaxis episode.1
References: 1. PALYNZIQ [package insert]. Novato, CA: BioMarin Pharmaceutical Inc; 2020. 2. Burton B, Sacharow S, Northrup H, et al. Efficacy and safety of the recommended pegvaliase dosing regimen in adults with phenylketonuria in the phase 3 PRISM studies. Poster presented at: SSIEM Annual Symposium; August 30-September 2, 2022; Freiburg, Germany. 3. Data on file. BioMarin Pharmaceutical Inc. 4. Thomas J, Levy H, Amato S, et al, for the PRISM investigators. Pegvaliase for the treatment of phenylketonuria: results of a long-term phase 3 clinical trial program (PRISM). Mol Genet Metab. 2018;124(1):27-38. doi:10.1016/j.ymgme.2018.03.006 5. Sacharow S, Northrup H, Whitehall KB, et al. Efficacy and safety of the recommended pegvaliase dosing regimen in adults with phenylketonuria in the phase 3 PRISM studies. Presented at the International Congress of Inborn Errors of Metabolism (ICIEM); November 21-24, 2021; Sydney, Australia.
BOXED WARNING: RISK OF ANAPHYLAXIS
WARNINGS AND PRECAUTIONS
Anaphylaxis
Other Hypersensitivity Reactions
ADVERSE REACTIONS
Blood Phenylalanine Monitoring and Diet
DRUG INTERACTIONS
Effect of PALYNZIQ on Other PEGylated Products
USE IN SPECIFIC POPULATIONS
Pregnancy and Lactation
Pediatric Use
Geriatric Use
You are encouraged to report suspected adverse reactions to BioMarin at 1-866-906-6100, or to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, with Boxed Warning for risk of anaphylaxis, and Medication Guide here.
INDICATION
PALYNZIQ is a phenylalanine (Phe)-metabolizing enzyme indicated to reduce blood Phe concentrations in adult patients with phenylketonuria who have uncontrolled blood Phe concentrations greater than 600 micromol/L on existing management.