In the randomized, double‐blind, multicenter, placebo‐controlled clinical trial, 39 patients with MPS VI received 1 mg/kg NAGLAZYME® (galsulfase) or placebo once‐weekly for 24 weeks. Patients were then enrolled in a 72-week open-label extension study, for a total of up to 96 weeks. Enrollment was restricted to patients with a 12‐minute walk distance of 5 to 400 meters. All patients were treated with antihistamines prior to each infusion. Results from the 10-year Resurvey Study, which evaluated patients who were involved in the initial Survey Study, are consistent with the phase 3 trial results.1-3
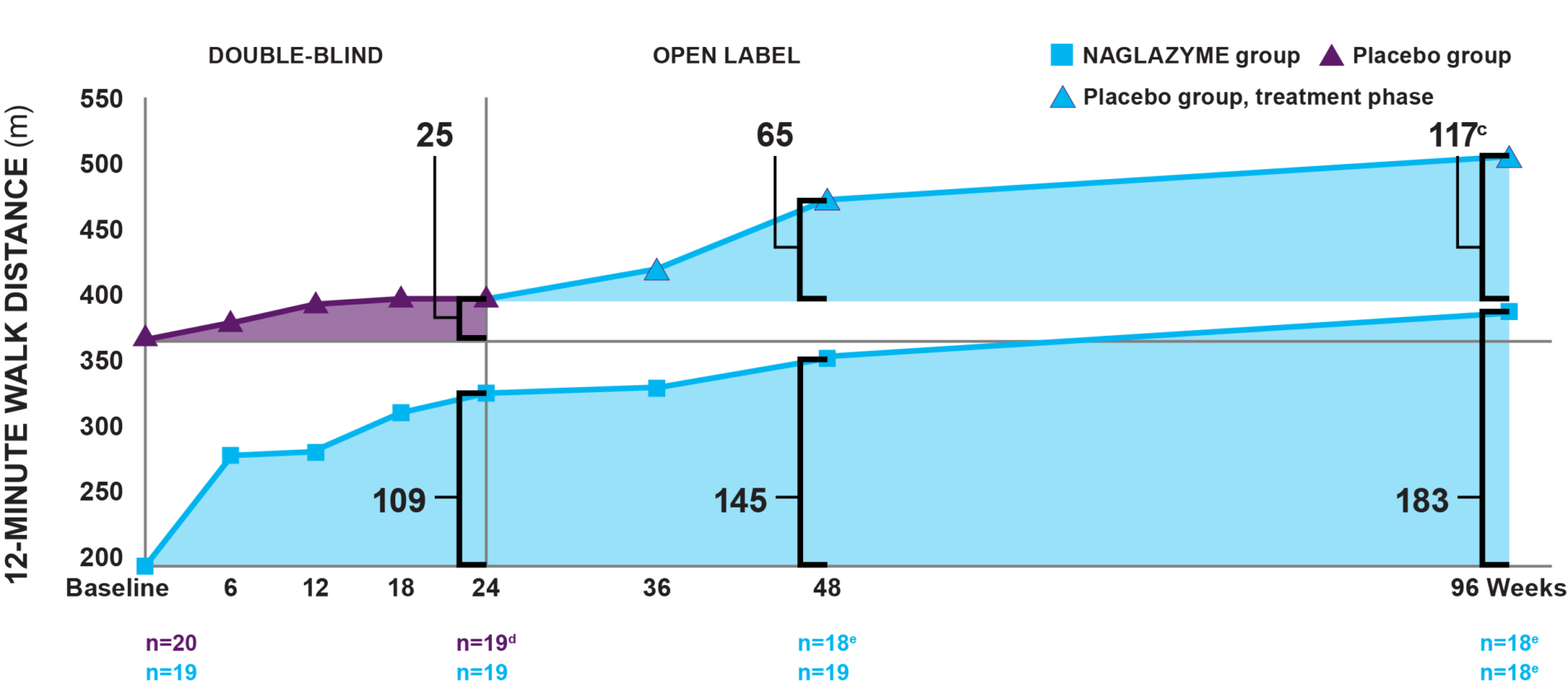
a Fitted values.1
b The difference between the NAGLAZYME and placebo groups at baseline was statistically significant (P=0.014).1
c By week 96, patients in the drug group had been taking NAGLAZYME for 96 weeks; patients in the placebo group had only been taking NAGLAZYME for the 72-week open-label extension period.2,4
d One patient in the placebo group left the study for reasons unrelated to treatment. Patients receiving placebo were switched to NAGLAZYME in the trial extension period.1,2
e One patient failed to complete the assessment.2
Week 24: Patients treated with NAGLAZYME demonstrated statistically and clinically significant improvement in endurance compared to placebo as measured by a 12MWT at week 24.1
Week 96: In the group originally treated with NAGLAZYME, improvement in the 12MWT was sustained through week 96.2
As exemplified by these clinical trial data, the walk test has emerged as a proven, trusted, and relatively simple method of measuring endurance in patients with MPS VI. The test demonstrates how performance impairment is a measure of disease progression.5
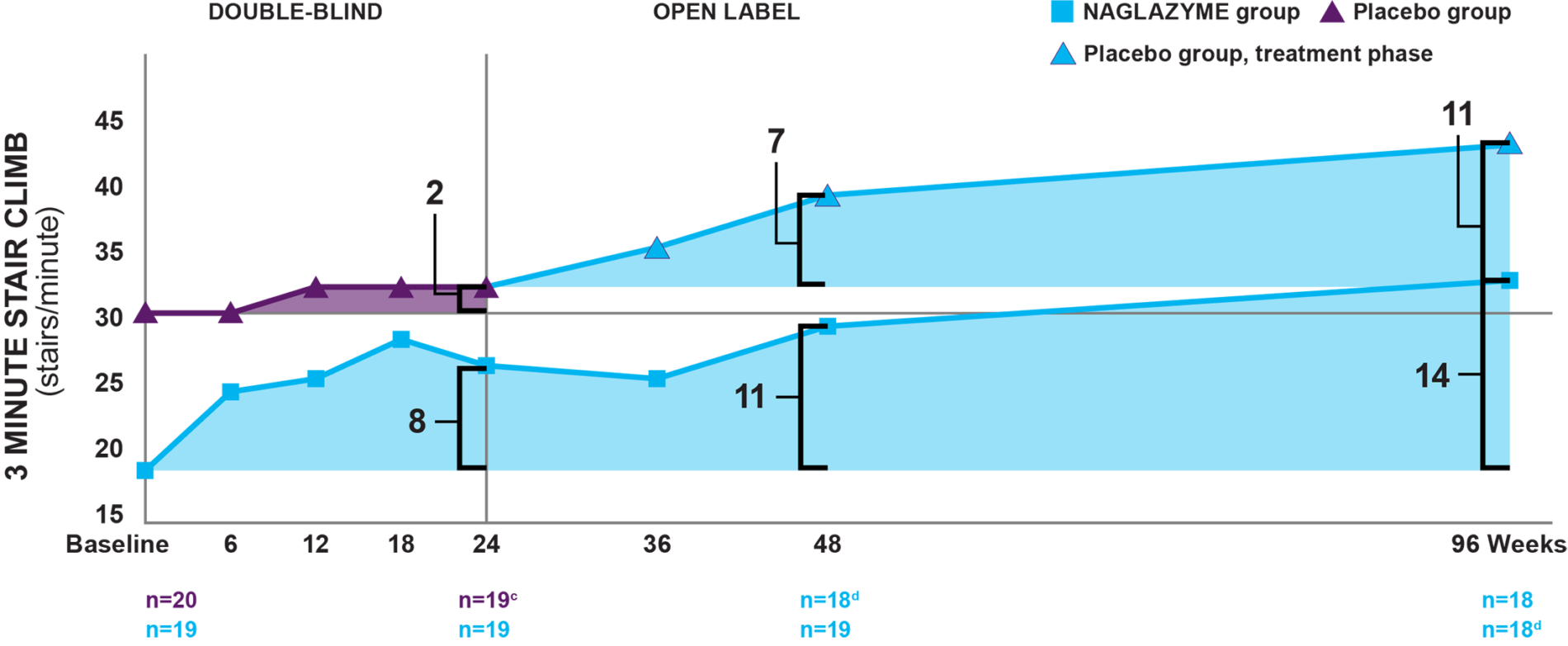
a Fitted values.1
b By week 96, patients in the drug group had been taking NAGLAZYME for 96 weeks; patients in the placebo group had only been taking NAGLAZYME for the 72-week open-label extension period.2
c One patient in the placebo group left the study for reasons unrelated to treatment. Patients receiving placebo were switched to NAGLAZYME in the trial extension period.1
d One patient failed to complete the assessment.2
Week 24: Patients treated with NAGLAZYME demonstrated improvement in the 3‐minute stair climb compared to placebo at week 24, though statistical significance was not reached (P=0.053).1,4
Week 96: In the group originally treated with NAGLAZYME, improvement in the rate of stair‐climbing was demonstrated at week 96.2
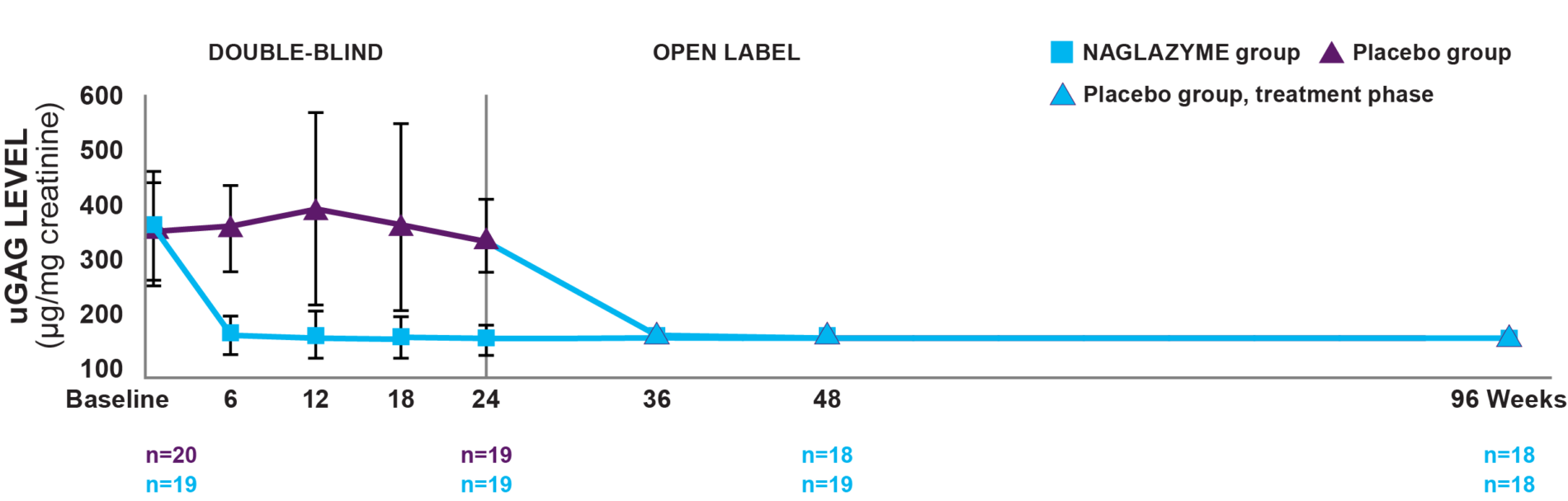
Week 24: In patients treated with NAGLAZYME, mean uGAG levels were reduced by 75% at week 24, a significantly greater reduction than patients on placebo (P<0.001).1
Week 96: In the group originally treated with NAGLAZYME, the reduction in uGAG levels was sustained through week 96. From week 48 to week 96, no change in uGAG levels was observed in the NAGLAZYME group.2
Overall, 95% of patients showed at least a 50% reduction in uGAG levels after 72 weeks of treatment with NAGLAZYME.4
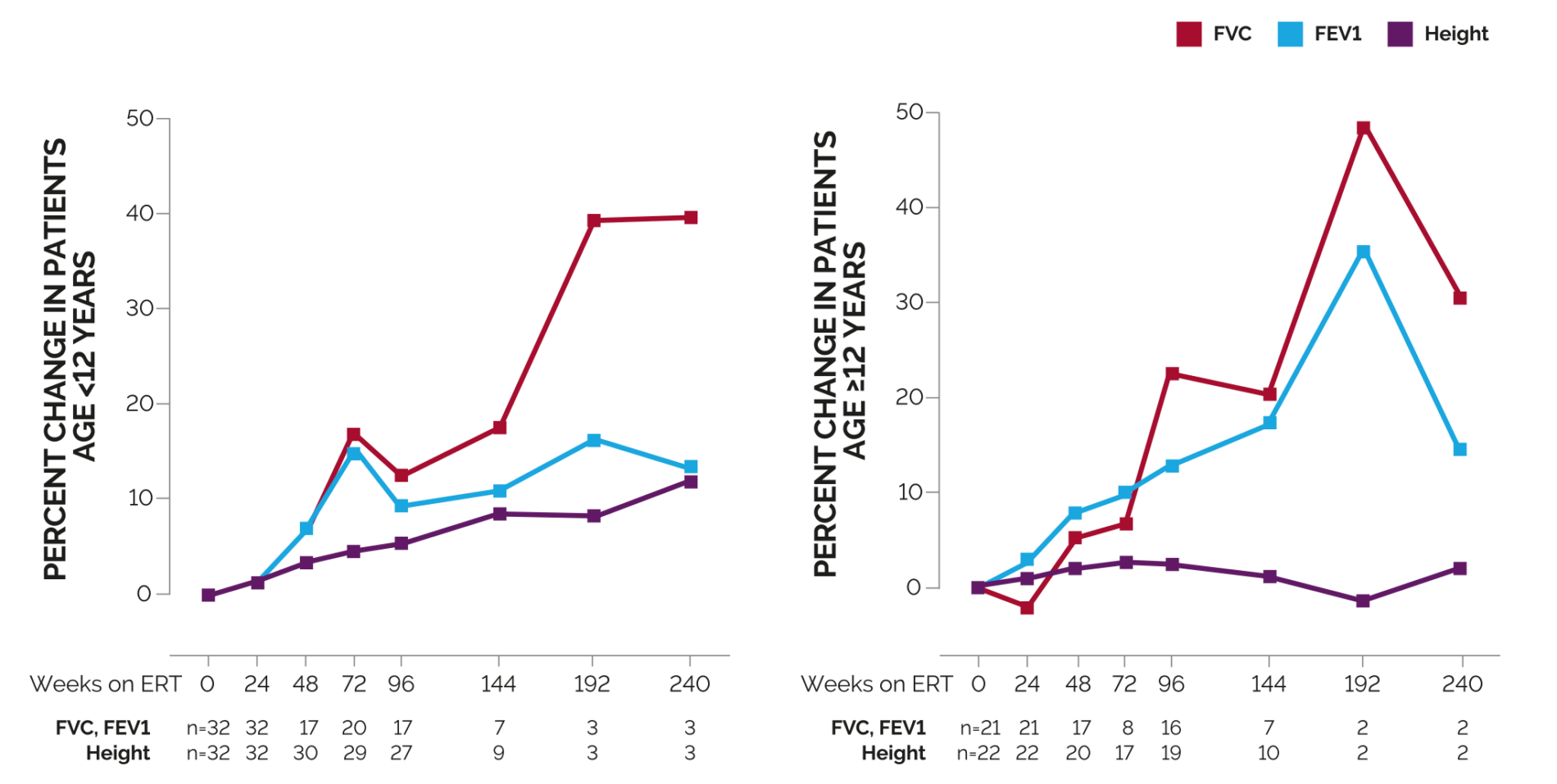
Week 96: By 96 weeks of treatment, increases in forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) averaged approximately 10% and 13%, respectively, for patients <12 years of age.6
Regardless of phenotype or age, NAGLAZYME improves pulmonary function over the long-term3
INDICATION AND IMPORTANT SAFETY INFORMATION
WARNING: HYPERSENSITIVITY REACTIONS INCLUDING ANAPHYLAXIS
Patients treated with enzyme replacement therapies have experienced life-threatening hypersensitivity reactions, including anaphylaxis. These reactions have occurred during and up to 24 hours after completion of the NAGLAZYME infusion. Anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy.
Administration of NAGLAZYME should be supervised by a healthcare provider knowledgeable in the management of hypersensitivity reactions, including anaphylaxis.
Initiate NAGLAZYME in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment. If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, discontinue NAGLAZYME and immediately initiate appropriate medical treatment, including use of epinephrine. In patients who have experienced anaphylaxis or other severe allergic reactions during infusion with NAGLAZYME, caution should be exercised upon rechallenge. Inform patients of the symptoms of life-threatening hypersensitivity reactions, including anaphylaxis and to seek immediate medical care should symptoms occur.
Immune-Mediated Reactions
As with other enzyme replacement therapies, immune-mediated reactions, including membranous glomerulonephritis have been observed. If immune-mediated reactions occur, consider discontinuing Naglazyme administration and initiate appropriate medical treatment. Consider the risks and benefits of re-administering Naglazyme following an immune-mediated reaction. Some patients have successfully been rechallenged and have continued to receive Naglazyme under close clinical supervision.
Risk of Acute Cardiorespiratory Failure
Caution should be exercised when administering NAGLAZYME to patients susceptible to fluid volume overload because congestive heart failure may result. Consider a decreased total infusion volume and infusion rate when administering NAGLAZYME to these patients.
Acute Respiratory Complications Associated with Administration
Consideration to delay NAGLAZYME infusion should be given when treating patients who present with an acute febrile or respiratory illness. Sleep apnea is common in MPS VI patients and antihistamine pretreatment may increase the risk of apneic episodes. Evaluation of airway patency should be considered prior to the initiation of treatment. Patients using supplemental oxygen or continuous positive airway pressure (CPAP) during sleep should have these treatments readily available during infusion in the event of an infusion reaction, or extreme drowsiness/sleep induced by antihistamine use.
Infusion Reactions
Pretreatment with antihistamines with or without antipyretics is recommended prior to the start of infusion to reduce the risk of infusion reactions. If infusion reactions occur, decreasing the infusion rate, temporarily stopping the infusion, or administering additional antihistamines and/or antipyretics is recommended.
Spinal or Cervical Cord Compression
Spinal/cervical cord compression is a known and serious complication that is expected to occur during the natural course of MPS VI. Signs and symptoms of spinal/cervical cord compression include back pain, paralysis of limbs below the level of compression, and urinary or fecal incontinence. Patients should be evaluated for spinal/cervical cord compression prior to initiation of NAGLAZYME to establish a baseline and risk profile. Patients treated with NAGLAZYME should be regularly monitored for the development or progression of spinal/cervical cord compression and be given appropriate clinical care.
Adverse Reactions
During infusion, serious adverse reactions included laryngeal edema, apnea, pyrexia, urticaria, respiratory distress, angioedema, and anaphylactoid reaction; severe adverse reactions included urticaria, chest pain, rash, abdominal pain, dyspnea, apnea, laryngeal edema, and conjunctivitis. The most common adverse events (≥10%) observed in clinical trials in patients treated with NAGLAZYME were rash, pain, urticaria, pyrexia, pruritus, chills, headache, nausea, vomiting, abdominal pain and dyspnea. The most common adverse reactions requiring interventions are infusion-related reactions.
To report SUSPECTED ADVERSE REACTIONS, contact BioMarin Pharmaceutical Inc. at 1-866-906-6100, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information with Boxed Warning for risk of anaphylaxis or visit www.Naglazyme.com.
What is NAGLAZYME used for?
NAGLAZYME® (galsulfase) is indicated for patients with mucopolysaccharidosis VI (MPS VI; Maroteaux-Lamy syndrome). NAGLAZYME has been shown to improve walking and stair-climbing capacity.